- Language
- 🇺🇸
- Joined
- Jul 7, 2022
- Messages
- 35
- Reaction score
- 14
- Points
- 8
Ahhhh. Just the sound of those syllables, so magically assembled into one harmonious whole. Just the name brings a wondrous and interesting series of images. Images.... ahh, er., where was I?
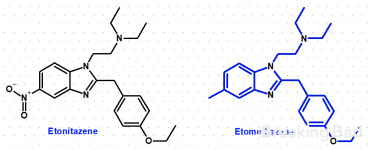
Oh yeah. Sorry about that. I want to synthesize etomethazene. That is etonitazene with a methyl group in place of the original nitro:

There are 3 or more different synthetic routes. For me, the one that makes the most sense, primarily because he precursors seem to be the easiest to procure or synthesize. This route starts with 3,4-diaminotoluene reacting with 4-ethoxyphenylacetonitrile to give the substituted benzimidazole (2-[(4-ethoxyphenyl)methyl]-5-methyl-2,3-dihydro-1H-benzimidazole):

The reaction of the benzimidazole reaction product with 2-chloroethyl-N,N-diethylamine gives the base etomethazene:

The precursor 4-ethoxyphenylacetonitrile, I believe, can be synthesized starting with the amino acid tyrosine, which is
decarboxylated to give tyramine:

The tryamine is then esterified with EtOH to give 2-(4-ethoxyphenyl)ethylamine.

Finally, the primary amine is oxidized to the nitrile with the pool chemical trichloroisocyanuric acid:

I am pretty sure that this precursor route is the most direct from an OTC point of view.
I am open to criticism and any questions you all may have.
I am going to post my views on the synthesis of the 2-chloroethyl-N,N-diethylamine, starting with diethylamine and dichloroethane (or dibromo ?).
plancklong
Oh yeah. Sorry about that. I want to synthesize etomethazene. That is etonitazene with a methyl group in place of the original nitro:
There are 3 or more different synthetic routes. For me, the one that makes the most sense, primarily because he precursors seem to be the easiest to procure or synthesize. This route starts with 3,4-diaminotoluene reacting with 4-ethoxyphenylacetonitrile to give the substituted benzimidazole (2-[(4-ethoxyphenyl)methyl]-5-methyl-2,3-dihydro-1H-benzimidazole):
The reaction of the benzimidazole reaction product with 2-chloroethyl-N,N-diethylamine gives the base etomethazene:
The precursor 4-ethoxyphenylacetonitrile, I believe, can be synthesized starting with the amino acid tyrosine, which is
decarboxylated to give tyramine:
The tryamine is then esterified with EtOH to give 2-(4-ethoxyphenyl)ethylamine.
Finally, the primary amine is oxidized to the nitrile with the pool chemical trichloroisocyanuric acid:
I am pretty sure that this precursor route is the most direct from an OTC point of view.
I am open to criticism and any questions you all may have.
I am going to post my views on the synthesis of the 2-chloroethyl-N,N-diethylamine, starting with diethylamine and dichloroethane (or dibromo ?).
plancklong