Procedure:
32 g tosylic acid monohydrate was dissolved in 150 mL water and refluxed with 8 g melatonin in a round-bottom flask for 9 hours. The mixture was decanted, slowly basified with 12 g NaOH in an ice bath, and refrigerated until clear. The precipitate was filtered, washed twice with cold concentrated ammonia, and dried under vacuum to obtain 4.444 g white powder (67%).
Characterization:
Notes:
32 g tosylic acid monohydrate was dissolved in 150 mL water and refluxed with 8 g melatonin in a round-bottom flask for 9 hours. The mixture was decanted, slowly basified with 12 g NaOH in an ice bath, and refrigerated until clear. The precipitate was filtered, washed twice with cold concentrated ammonia, and dried under vacuum to obtain 4.444 g white powder (67%).
Characterization:
- Positive reaction (purple color development) with 0.2% ethanolic ninhydrin, which points at a primary (or secondary) amine.
- Melting point: 121.4 °C (121.5 °C in literature)
- Poorly soluble in neutral and basic water, soluble in acidic water. Somewhat soluble in ethanol. Very soluble in acetone, methanol, DCM, and THF. Soluble in hot acetonitrile.
- Tosylate salt recrystallizes well from ethyl acetate. Very soluble in hot and/or acidic water. Somewhat soluble in DCM, poorly soluble in THF, almost insoluble in acetone.
- Like melatonin, mexamine absorbs 264 nm ultraviolet light. Unlike melatonin, it is NOT luminescent under 365 nm light.
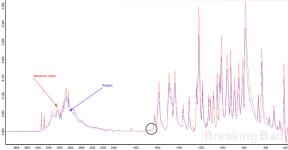
- The IR spectrum has two peaks at 3335 cm⁻¹ and 3283 cm⁻¹ that correspond to stretching of the two N–H bonds of a primary amine. Overall the spectrum is nearly identical to that of retail mexamine (attached), with a tiny anomaly in the 1650 cm⁻¹ region. Peaks in that region correspond to the stretching of the C=O carbonyl group in amides, which means there are trace amounts of melatonin in the product. It seems unnecessary to purify it since melatonin will be lost in subsequent reactions.
Notes:
- Using phosphoric and sulfuric instead of tosylic acid led to substantial contamination of the product with brown gunk.
- The reaction was not optimized — it is possible that the amount of tosylic acid and NaOH can be reduced.
- Acidic hydrolysis is a reversible reaction, so it does not run to completion. Boiling for more than 8–10 hours is unlikely to produce much higher yields. The only ways to increase the yield seem to be continuous distillation of acetic acid out of the reaction mixture, or continuous addition of methanol and distilling low-boiling methyl acetate out.
- While poorly soluble in neutral water, melatonin becomes soluble in acidic or basic water, coloring it yellow.