- Joined
- Jun 24, 2021
- Messages
- 1,644
- Solutions
- 2
- Reaction score
- 1,709
- Points
- 113
- Deals
- 666
General information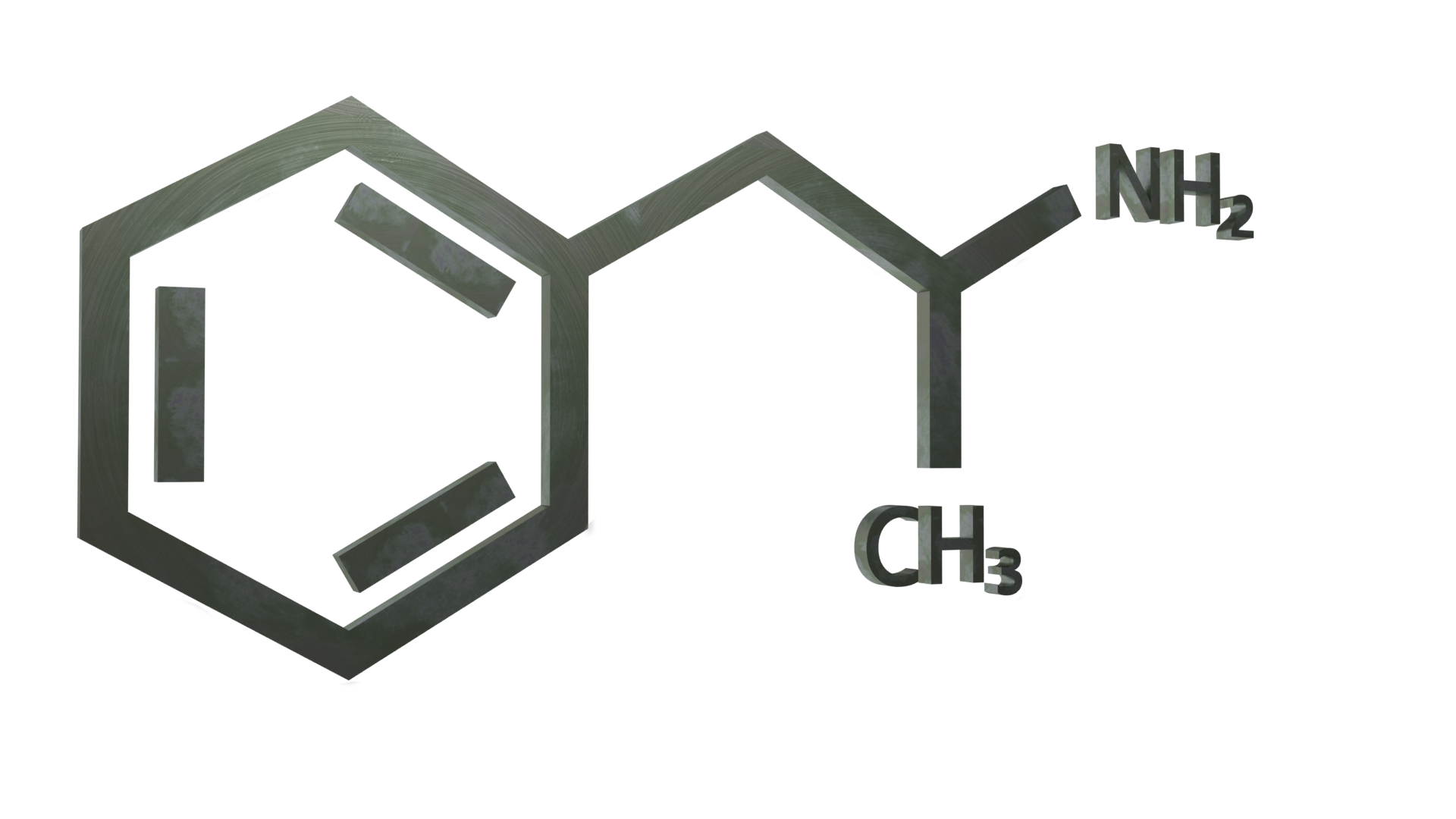
Amphetamine (also known as alpha-methylphenethylamine, amfetamine, and speed) is a classical stimulant substance of the phenethylamine class. It is the parent compound of the substituted amphetamines, a diverse group that includes methamphetamine, MDMA, cathinone, and bupropion. The mechanism of action involves promoting release of the neurotransmitters dopamine and norepinephrine.
Amphetamine, a substance discovered over 100 years ago, is one of the most restricted controlled drugs. It was previously used for a large variety of conditions and this changed until this point where its use is highly restricted. Amphetamine, with the chemical formula alpha-methylphenethylamine, was discovered in 1910 and first synthesized by 1927. After being proven to reduce drug-induced anesthesia and produce arousal and insomnia, amphetamine racemic mix was registered by Smith, Kline and French in 1935. Amphetamine structure presents one chiral center and it exists in the form of dextro- and levo-isomers. The first product of Smith, Kline and French was approved by the FDA on 1976.
In the 1930s, it was sold over-the-counter under the name "Benzedrine" as a decongestant. It became widely used to treat a range of ailments such as alcohol hangover, narcolepsy, depression, and obesity. During World War II, amphetamine was used to promote wakefulness in the soldiers. This use derived into a large overproduction of amphetamine and all the surplus after the war finalized ended up in the black market, producing the initiation of the abuse. Due to issues with addiction and abuse, it was eventually listed as a controlled substance under the United Nations 1971 "Convention on Psychotropic Substances".
Amphetamine is now primarily a prescription drug used to treat attention deficit hyperactivity disorder (ADHD), narcolepsy, and obesity. Additionally, it sees widespread illicit use as a performance enhancing agent and recreational substance.
Physical properties
- Formula C9H13N
- Molar mass 135.210 g/mol
- Density 0.936 g/cm3 at 25 °C
- Melting point 11.3 °C (52.3 °F)
- Boiling point 200-203 °C (397 °F) at 760 mmHg
Chemical properties
The free base of amphetamine is a colorless volatile oily liquid with a characteristic "fishy" odor and acrid, burning taste, poorly soluble in water, readily soluble in organic solvents, boiling point 200-203 °C.
Amphetamine is a methyl homolog of the mammalian neurotransmitter phenethylamine with the chemical formula C9H13N. The carbon atom adjacent to the primary amine is a stereogenic center, and amphetamine is composed of a racemic 1:1 mixture of two enantiomers. This racemic mixture can be separated into its optical isomers: levoamphetamine and dextroamphetamine (l- and d- isomers). Frequently prepared solid salts of amphetamine include amphetamine hydrochloride, phosphate, sulfate. Dextroamphetamine sulfate is the most common enantiopure salt. Amphetamine is also the parent compound of its own structural class, which includes a number of psychoactive derivatives.
Synthesis ways
There are list of most popular amphetamine synthesis ways. All of them have own advantages and disadvantages. Most popular non-selective synthesis is P2NP reduction, which can be carried out with aluminium (Al) amalgam. Also, it is possible to reduce by NaBH4, LAH or hydrogen gas with catalyst (PtO2 or Pd/C) and excess pressure. P2NP can be synthesized by simple condensation of nitroethane with benzaldehyde.
One of the most common methods of clandestine amphetamine production is the Leuckart reaction, which consists of the condensation of phenylacetone (phenyl-2-propanone, P2P) with formamide or ammonium formate in the presence of formic acid and subsequent acid hydrolysis of the resulting N-formylamphetamine.
A mphetamine can also be prepared by reductive amination of phenylacetone (P2P) in the presence of a metal catalyst. The reaction proceeds with the formation of an intermediate imine. Examples of a reaction are: Heterogeneous catalytic reduction of phenylacetone with ammonia. The catalyst may be palladium on carbon, platinum oxide or Raney nickel. Restoration with aluminum, zinc or magnesium amalgams.
If necessary, the amphetamine stereoisomers dextroamphetamine and levoamphetamine can be separated using tartaric acid. In addition, a method has been published for the stereoselective synthesis of dextroamphetamine, which consists in the reductive amination of phenylacetone with S-α-methylbenzylamine. The imine, which was obtained, is reduced with Pd/C or Raney nickel and recrystallized as the hydrochloride. The N-benzyl group is then hydrogenolyzed in the presence of palladium on charcoal to form high optical purity dextroamphetamine.
Analysis and purification
Toxic and dangerous substances are used in any synthesis way of amphetamine. There are two amphetamine purification methods "Product Washing" and more advanced method "Acid-base Extraction".
Drug washing is an essential and final part of almost any synthesis. Sometimes repeated several times. The method is available to anyone, does not require skills, can significantly improve the quality of product and presentation. The method is Ideal for small quantities. Washing is indicated for residues of P2NP, alkalis, acids and so on. Washing will not remove contaminants (acetaminophen, caffeine, etc.) and mercury salts.
The most accessible, and therefore easier, is to wash amphetamine with isopropyl alcohol (IPA). More difficult to use is anhydrous acetone. IPA does not contain water, and therefore it does not dissolve amph salt. The key to the process success is the lack of water. It is needing for avoiding amph from dissolution with pollutants because they will be thrown out.
Acid-base extraction (ABE), as a purification method, allows you to get a high-quality drug. Method is good by reason of using available reagents, tools and instruments.
Amphetamine is cut unacceptably often by caffeine, starch, nootropics such as Cinnarizine and Piracetam, a-PVP, methamphetamine and other stimulants and pharmacy substances. There are several methods to check your amphetamine. The most popular and easiest way is Drugs testing reagents. You can read about other methods in Amphetamine assessment protocol.
There are pictures of different amphetamine samples after tests by reagents
Effects and dosage
Subjective effects include stimulation, focus enhancement, motivation enhancement, increased libido, appetite suppression, and euphoria. It is usually taken orally, but can also be insufflated, injected, or administered rectally. Lower doses tend to increase focus and productivity while higher doses tend to increase sociability, sexual desire, and euphoria.
Amphetamine has high abuse potential. Chronic use (i.e. high dose, repeat administration) is associated with compulsive redosing, escalating tolerance, and psychological dependence. Additionally, abuse has been linked to a number of health conditions, especially cardiovascular issues such as high blood pressure and increased risk of stroke. It is highly advised to use harm reduction practices if using this substance.
[SPOILER=Physical effects]
Stimulation - Amphetamine is reported to be very energetic and stimulating. It can encourage physical activities such as dancing, socializing, running, or cleaning. The particular style of stimulation that amphetamine produces can be described as forced. This means that at higher dosages, it becomes difficult or impossible to keep still. Jaw clenching, involuntary bodily shakes, and vibrations become present, resulting in extreme shaking of the entire body, unsteadiness of the hands, and a general loss of fine motor control. This is replaced with mild fatigue and general exhaustion during the offset of the experience.
-
Spontaneous bodily sensations - The "body high" of amphetamine can be described as a moderate euphoric tingling sensation that encompasses the entire body. This sensation maintains a consistent presence that steadily rises with the onset and hits its limit once the peak has been reached.
-
Physical euphoria
-
Abnormal heartbeat
-
Increased heart rate
-
Increased blood pressure- By about 30mmHg systolic and 20mmHg diastolic, from naive users taking 40mg d-AMP.
-
Appetite suppression
-
Bronchodilation
-
Dehydration
-
Dry mouth
-
Frequent urination
-
Difficulty urinating
-
Increased bodily temperature
-
Increased perspiration
-
Mania - Amphetamine can produce mania in genetically predisposed individuals, such as those on the spectrum of bipolar disorder or schizophrenia. Higher doses and sleep deprivation appears to increase the risk.
-
Nausea - This can be mitigated by eating before dosing and throughout the experience.
-
Pupil dilation - This effect is experienced only at common to high dosages and is more prominent on the comedown.
-
Reflex syncope
-
Stamina enhancement
-
Teeth grinding - Teeth grinding may be present at higher doses. However, it is less intense than that of MDMA.
-
Temporary erectile dysfunction
-
Vasoconstriction - Amphetamine use causes blood vessels to constrict resulting in not enough blood reaching some parts of the body. This can cause feelings of tingling or pain, a cold feeling, numbness, paleness, or skin color changes especially in the fingers and toes.
[/SPOILER]
[SPOILER=Visual effect]
-
The visual effects of amphetamine are inconsistent and occur only mildly noticeable at higher doses. They are somewhat comparable to deliriant visuals and occur more readily in darker areas.
[/SPOILER]
[SPOILER=Distortions]
-
Drifting - This effect is usually subtle and barely noticeable and only occurs at higher dosages or when combined with cannabis. Commonly this consists of level 1-2 drifting.
- Brightness alteration - Amphetamine can make spaces seem brighter as a result of its pupil dilating effects.
- Tracers - This effect is imperceptible with low dosages. It's most pronounced with bigger dosages and especially when someone becomes sleep deprived, what on the other hand can be easily provoked by other effects of this substantion. Transformations - This effect occurs very rarely, and typically only when the user has taken high doses, is coming down, or has been awake for unusually long periods. They are usually very mild when they do occur.
[/SPOILER]
[SPOILER=Hallucinatory states]
-
Transformations - This effect occurs very rarely, and typically only when the user has taken high doses, is coming down, or has been awake for unusually long periods. They are usually very mild when they do occur.
-
Geometry - This effect is reported by some users of amphetamine and related substances, typically at heavier doses when one is attempting to sleep. It can be described in its variations as simplistic, algorithmic, synthetic, dimly lit, multicolored, glossy, sharp edges, zoomed out, smooth, angular, immersive, and progressive. It typically occurs at level 3 however may progress to 4 and 5 when combined with substances like cannabis or DXM.
[/SPOILER]
[SPOILER=Cognitive effects]
- Analysis enhancement
- Cognitive euphoria
- Compulsive redosing
- Ego inflation
- Emotion suppression - This effect is typically most intense at light and common doses, and is more commonly reported from medical usage rather than recreational.
- Focus enhancement - This effect is most effective at low to moderate doses as anything higher will usually impair concentration.
- Increased libido - While amphetamine use can cause feelings of sexual enhancement, the constricting of blood vessels may make it difficult to get or maintain an erection.
- Increased music appreciation
- Irritability - This is more likely to occur at higher doses.
- Memory enhancement
- Motivation enhancement
- Psychosis - This effect only occurs in either predisposed individuals, or after chronic, high frequency use, or due to sleep deprivation.
- Suggestibility suppression
- Thought acceleration
- Thought organization
- Time distortion - This can be described as the experience of time speeding up and passing much quicker than it usually would when sober.
- Wakefulness
[/SPOILER]
[SPOILER=After effects]
The effects which occur during the offset of a stimulant experience generally feel negative and uncomfortable in comparison to the effects which occurred during its peak. This is often referred to as a "comedown" and occurs because of neurotransmitter depletion. Its effects commonly include:
- Anxiety - Anxiety can reach severe levels during the comedown in some users.
- Appetite suppression
- Cognitive fatigue
- Depression
- Increased heart rate - While blood concentration of amphetamine and most subjective effects are highest about 3 hours after administration, heart rate peaks much later at 10 hours after administration.
- Irritability
- Motivation suppression
- Restless legs
- Sleep paralysis - Some users note sleep paralysis after consuming amphetamine.
- Dream suppression
- Thought deceleration
- Wakefulness - The insomnia following a repeated series of amphetamine doses can last for longer than a day in some users.
- Motivation suppression - Experiences can range from mild demotivation to extreme states of disinterest. This effect is more prominent at common and heavy doses.
[/SPOILER]
Pharmacology
Amphetamine exerts its behavioural effects by increasing the signaling activity of neurotransmitters norepinephrine and dopamine in the reward and executive function pathways of the brain. The reinforcing and motivational effects of amphetamine are mostly due to enhanced dopaminergic activity in the mesolimbic pathway.
The euphoric and locomotor-stimulating effects of amphetamine are dependent upon the magnitude and speed by which it increases synaptic dopamine and norepinephrine concentrations in the striatum.
It is a potent full agonist of the trace amine-associated receptor 1 (TAAR1) and interacts with vesicular monoamine transporter 2 (VMAT2). Combined action on TAAR1 and VMAT2 results in increased concentrations of dopamine and norepinephrine in the synapses, which stimulates neuronal activity.
Dextroamphetamine is a more potent agonist of TAAR1 than levoamphetamine. Consequently, dextroamphetamine produces greater CNS stimulation than levoamphetamine, roughly three to four times more, but levoamphetamine has slightly stronger cardiovascular and peripheral effects.
The exact bioavailability of amphetamine is not known, but it is believed to be over 75% by mouth, and higher by injection or intranasal administration. Its absorption and excretion may be pH dependent. As it is a weak base hence the more basic the environment the more of the drug is found in a lipid-soluble form and the absorption through lipid-rich cell membranes is highly favored. The peak response of amphetamine occurs 1-3 hours after oral administration and approximately 15 minutes after injection. Complete amphetamine absorption is usually done after 4-6 hours. The basic form is more readily absorbed in the intestine and less readily removed by the kidneys, potentially increasing its half life. It is removed by the kidneys via excretion and a small amount is removed by hepatic enzymes.
Ilegal market data
Global supply of amphetamine-type stimulants (ATS)
A record quantity of over 525 tons of ATS was seized in 2020, which represents a 15 per cent increase year on year 1 and continued the upward trend observed over the period 2010–2020. The quantities of methamphetamine seized grew five fold over that 10-year period, the quantities of amphetamine seized almost quadrupled and the quantities of “ecstasy” seized more than tripled.
Use of amphetamines continued to rise but signs of decrease in demand for treatment in 2020. Primarily on the basis of self-reported responses to general population surveys, a total of 34 million people aged 15–64, or 0.7 per cent of the global population, are estimated to have used amphetamines in the past year, and 20 million (0.4 per cent) are estimated to have used “ecstasy”-type substances. Some of those users had used both types of substances. The two most commonly used amphetamines are amphetamine and methamphetamine.
The global estimate of amphetamines use was similar in 2010, with 33 million past year users or 0.7% of the population aged 15-64. However, these estimates have to be interpreted with caution owing to the lack of data from major consumer countries in Asia where other market indicators, such as seizures and prices, suggest an expansion over the last decade. Qualitative information based on perceptions of trends reported by national experts to UNODC shows a continued increase both in terms of the use of amphetamines and the number of people in treatment for amphetamines over the past decade. However, data for 2020 show that this increasing trend has paused and that the number of people in treatment for amphetamines may have decreased, consistent with an overall decrease in treatment as a result of the COVID-19 pandemic. e Trends derived from such qualitative information are consistent with the available supply indicators, such as prices and seizures, which indicate continued global expansion of the market for amphetamines. Qualitative information of this type suffers from methodological limitations, but it has an advantage in that it takes into consideration small-scale studies and expert observations regarding countries where drug use surveys are not regularly implemented. Qualitative information on trends in the use of “ecstasy” was reported under different categories by countries before the implementation by UNODC of its new data collection tool (the updated annual report questionnaire, which came into use in 2020), thus qualitative reports of trends in “ecstasy” use are limited to the period 2019–2020. These reports suggest a moderate increase globally. At the same time, studies from countries where “ecstasy” is used in recreational settings suggest that the use of “ecstasy” declined more than any other drug during the pandemic in those countries. Wastewater analysis, while limited in geographical coverage to Europe, North America and some parts of Asia and Oceania, also suggests that the use of “ecstasy” declined between 2019 and 2020 more than the use of amphetamines. In the majority of analyzed locations, increased levels of consumption of MDMA were identified, while in a slight majority of those locations, increased amphetamine use and decreased methamphetamine use were detected. Early wastewater analysis data from 2021 suggest an overall increase in amphetamine consumption in the majority of locations monitored by the Sewage Analysis CORe group, most of which are in Europe, between 2020 and 2021; an increase and a decrease in methamphetamine consumption in about the same number of locations; and a continuous decrease in MDMA consumption in a large majority of locations.
