WillD
Expert
- Joined
- Jul 19, 2021
- Messages
- 774
- Reaction score
- 1,061
- Points
- 93
Reagents:

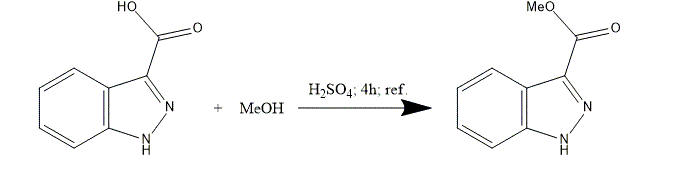
Methyl 1-pentyl-1H-indazole-3-carboxylate
 1-Pentyl-1H-indazole-3-carboxylic acid
1-Pentyl-1H-indazole-3-carboxylic acid
 AB-PINACA ((S)-N-(1-amino-3-methyl-1-oxobutan-2-yl)-1-pentyl-1H-indazole-3-carboxamide)
AB-PINACA ((S)-N-(1-amino-3-methyl-1-oxobutan-2-yl)-1-pentyl-1H-indazole-3-carboxamide)
- Indazole-3-carboxylic acid (cas 4498-67-3) 100 g;
- Methanol (MeOH) 2500 ml;
- Sulphuric acid conc. (H2SO4) 100 ml 98%;
- Ethyl acetate (EtOAc) 7500 ml;
- Sodium bicarbonate aqueous solution (NaHCO3) 1000 ml;
- Distilled water (H2O) ~8000 ml;
- Sodium chloride (NaCl) ~300 g;
- Magnesium sulphate (MgSO4);
- Tetrahydrofuran (THF) 1000 ml;
- Potassium tert-butoxide (t-BuOK) 70 g
- 1-Bromopentane 80 ml;
- Sodium hydroxide 1M aqueous solution (NaOH) 600 ml;
- Hydrochloric acid 1M aq. solution (HCl);
- Dimethylformamide (DMF) 1000 ml;
- EDC (cas 1892-57-5) 82 g;
- BuOH (cas 71-36-3) 58 g;
- DIPEA ( cas 7087-68-5) 180 g;
- L-valinamide (cas 4540-60-7) 100 g;
- Rround bottom flasks 1L and 5 L;
- Retort stand and clamp for securing apparatus;
- Top stirrer;
- Drip funnel 500 mL;
- Water bath;
- Separatory funnel;
- Glass rod and spatula;
- Laboratory grade thermometer;
- Laboratory scale (0.1-200 g is suitable);
- Measuring cylinders 100 mL and 1 L;
- Funnel;
- Filter paper;
- pH Indicator paper;
- Rotovap machine;
- Vacuum source;
- Buchner flask (large) and funnel;
- Beakers 1000 ml x2; 500 ml x2; 250 ml x2;
1. Indazole-3-carboxylic acid 100 g solution in MeOH 1500 ml and 5 l round bottom flask is treated with concentrated H2SO4 100 ml (98%).
2. The mixture is refluxed for 4 h.
3. After that, the mixture is concentrated with vacuum and dissolved in ethyl acetate (EtOAc) 2500 ml.
4. An organic phase is washed with saturated sodium bicarbonate aqueous solution (NaHCO3) 1000 ml, H2O 1000 ml and brine 1000 ml. Then, the mixture is dried over magnesium sulphate (MgSO4).
5. Ethyl acetate (EtOAc) solvent is evaporated under reduced pressure. Methyl 1H-Indazole-3-carboxylate 83 g is obtained as a white solid.
2. The mixture is refluxed for 4 h.
3. After that, the mixture is concentrated with vacuum and dissolved in ethyl acetate (EtOAc) 2500 ml.
4. An organic phase is washed with saturated sodium bicarbonate aqueous solution (NaHCO3) 1000 ml, H2O 1000 ml and brine 1000 ml. Then, the mixture is dried over magnesium sulphate (MgSO4).
5. Ethyl acetate (EtOAc) solvent is evaporated under reduced pressure. Methyl 1H-Indazole-3-carboxylate 83 g is obtained as a white solid.
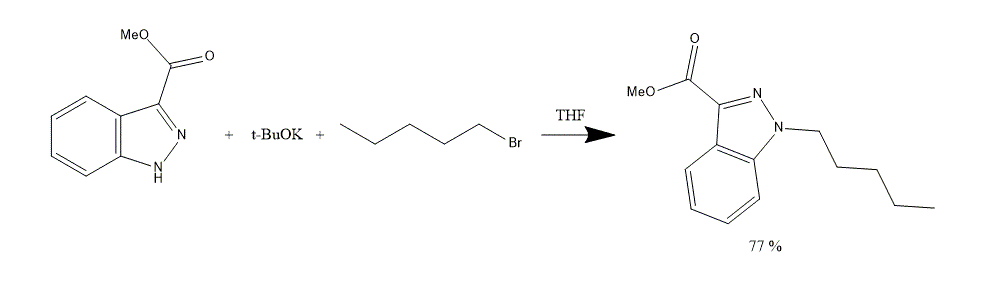
1. To a cooled Methyl 1H-Indazole-3-carboxylate 100 g, 0 °C solution in THF 1000 ml t-BuOK 70 g is added.
2. The mixture is warmed to a room temperature, stirred for 1 h and cooled to 0 °C. 1-Bromopentane 80 ml is added dropwise with a constant stirring.
3. The mixture is warmed to a room temperature, stirred for 48 h and distilled water 1000 mL is added.
4. Layers are separated. An aqueous layer is extracted with EtOAc 2x500 ml. Combined organic phase is washed with distilled water 3x500 ml and brine 1000 ml. Then, Organic solution is dried over MgSO4.
5. Solvent is evaporated under reduced pressure. Amethyl 1-pentyl-1H-indazole-3-carboxylate clear glass-like solid 67 g, 77% is obtained.
2. The mixture is warmed to a room temperature, stirred for 1 h and cooled to 0 °C. 1-Bromopentane 80 ml is added dropwise with a constant stirring.
3. The mixture is warmed to a room temperature, stirred for 48 h and distilled water 1000 mL is added.
4. Layers are separated. An aqueous layer is extracted with EtOAc 2x500 ml. Combined organic phase is washed with distilled water 3x500 ml and brine 1000 ml. Then, Organic solution is dried over MgSO4.
5. Solvent is evaporated under reduced pressure. Amethyl 1-pentyl-1H-indazole-3-carboxylate clear glass-like solid 67 g, 77% is obtained.
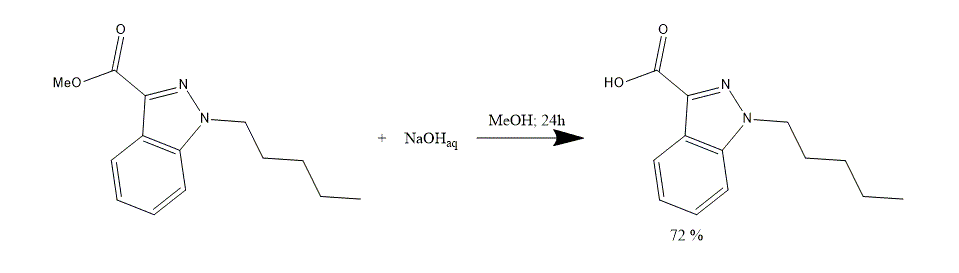
1. Methyl 1-pentyl-1H-indazole-3-carboxylate 100 g solution in MeOH 1000 ml is treated with sodium hydroxide 1M aqueous solution (NaOH) 600 ml and stirred for 24 h.
2. An organic solvent is evaporated in vacuum. A residue is dissolved in distilled water, acidified with hydrochloric acid 1M aq. solution (HCl) to pH 2-3 and extracted with EtOAc 2x500 ml.
3. The organic phase is dried over MgSO4 and the solvent is evaporated under reduced pressure. 1-Pentyl-1H-indazole-3-carboxylic acid 68 g, 72% is obtained as a white solid substance and used in a following synthesis step without purification.
2. An organic solvent is evaporated in vacuum. A residue is dissolved in distilled water, acidified with hydrochloric acid 1M aq. solution (HCl) to pH 2-3 and extracted with EtOAc 2x500 ml.
3. The organic phase is dried over MgSO4 and the solvent is evaporated under reduced pressure. 1-Pentyl-1H-indazole-3-carboxylic acid 68 g, 72% is obtained as a white solid substance and used in a following synthesis step without purification.
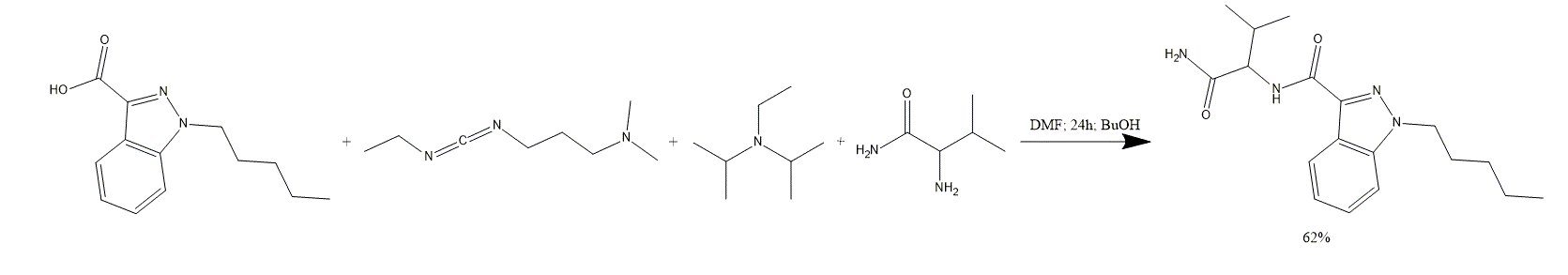
1. A 1-pentyl-1H-indazole-3-carboxylic acid 100 g solution in DMF 1000 ml is treated with EDC 82 g, BuOH 58 g, DIPEA 180 g, L-valinamide 100 g and stirred for 24 h.
2. The mixture is partitioned between and H2O 2000 ml and EtOAc 1000 ml. Layers are separated and the aqueous layer is extracted with EtOAc 2x1000 ml.
3. The combined organic phase is dried over MgSO4 and the solvent is evaporated under reduced pressure.
4. The crude AB-PINACA is purified by recrystallization. AB-PINACA 88 g is obtained as a white solid. The yield is 62%.
2. The mixture is partitioned between and H2O 2000 ml and EtOAc 1000 ml. Layers are separated and the aqueous layer is extracted with EtOAc 2x1000 ml.
3. The combined organic phase is dried over MgSO4 and the solvent is evaporated under reduced pressure.
4. The crude AB-PINACA is purified by recrystallization. AB-PINACA 88 g is obtained as a white solid. The yield is 62%.
Last edited by a moderator:
